Scientists have achieved a "striking" reversal of Alzheimer's disease in mice by restoring the normal function of the brain's vasculature -- the network of blood vessels that supplies it with oxygen and nutrients.
Researchers at the Institute for Bioengineering of Catalonia (IBEC) and West China Hospital of Sichuan University (WCHSU), working with partners in the UK, showed this was possible using nanotechnology.
"Our study demonstrated remarkable efficacy in achieving rapid Aβ clearance [in Alzheimer's the main 'waste' protein is amyloid-β], restoring healthy function in the blood-brain barrier [known as the BBB] and leading to a striking reversal of Alzheimer's pathology," said study author Lorena Ruiz Perez, researcher at the Molecular Bionics group from the IBEC, in a statement.
Typically, nanomedicine relies on nanoparticles as carriers for therapeutic molecules, while this approach uses nanoparticles that act as therapeutic agents in their own right, known as supramolecular drugs.
Instead of targeting neurons or other brain cells, the therapy restores the proper function of the BBB, the "vascular gatekeeper" that regulates the brain's environment, according to the researchers.
"By restoring this 'gatekeeper,' we help the brain rebalance itself and make any other therapy work better," Giuseppe Battaglia, Catalan Institution for Research and Advanced Studies (ICREA) research professor at IBEC, told Newsweek.
"Today, no treatment reliably reverses Alzheimer's; at best, some slow aspects of decline. Our results point to a new path: fix the barrier that keeps the brain healthy."
Battaglia explained a therapy that restores the brain's own defenses could mean "fewer day-to-day declines, longer periods of independence and better responses to existing medications for families, which could translate into more meaningful time together and a reduced caregiving burden."
The findings highlight the importance of vascular health and the link between diseases like dementia and Alzheimer's with a compromised vascular system.
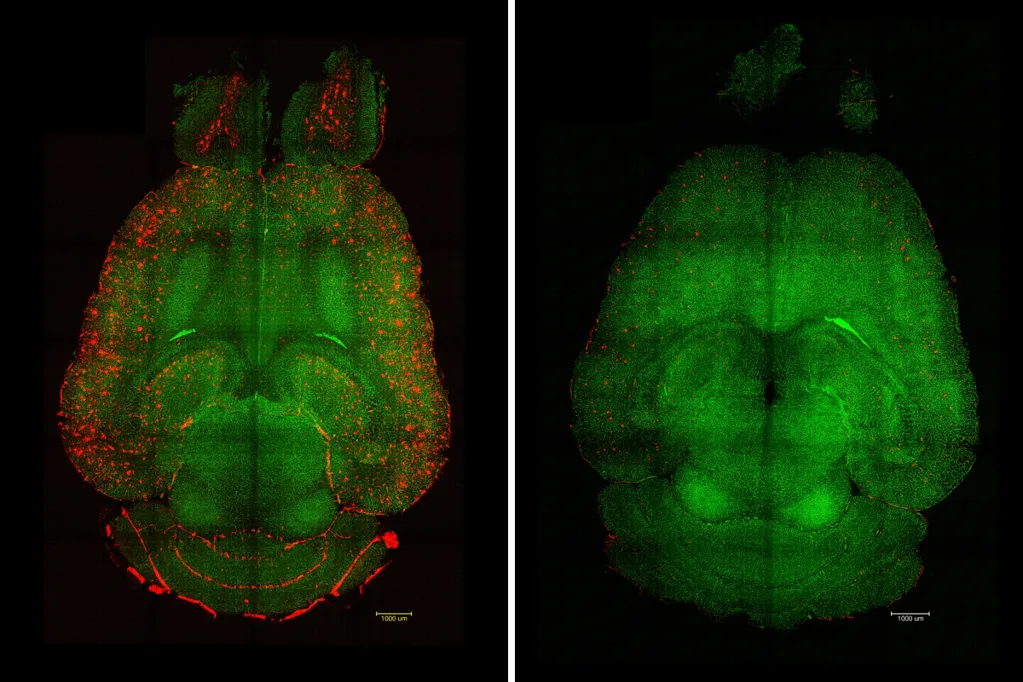
The team demonstrated that targeting a specific mechanism enabled undesirable "waste proteins" produced in the brain to pass through the BBB and be eliminated in the blood flow.
In Alzheimer's, an accumulation of Aβ affects the normal functioning of the neurons. The brain's natural clearance system for toxic species like this protein stops working properly.
In the study, the researchers used mouse models genetically programmed to produce larger amounts of Aβ and develop a significant cognitive decline mimicking Alzheimer's disease.
"Only 1h after the injection [of the supramolecular drugs] we observed a reduction of 50-60 percent in Aβ amount inside the brain," said study author Junyang Chen, researcher at WCHSU and University College London student.
"Animals show lasting functional recovery months later, suggesting durable benefits, not just a short-term effect," said Battaglia. "No damage or toxicity [was] observed in animals; they tolerate the therapy well."
In one experiment, they treated a 12-month-old mouse (equivalent to a 60-year-old human) with the nanoparticles. Analyzing its behaviour and memory after six months, they found the animal, then aged 18 months (comparable to a 90-year-old human), had recovered the behaviour of a healthy mouse.
"We think it works like a cascade: when toxic species such as amyloid-beta (Aβ) accumulate, disease progresses. But once the vasculature is able to function again, it starts clearing Aβ and other harmful molecules, allowing the whole system to recover its balance. What's remarkable is that our nanoparticles act as a drug and seem to activate a feedback mechanism that brings this clearance pathway back to normal levels," explained Chen.
By imitating the way natural molecules interact with the LRP1 receptor (which usually acts as a "molecular gatekeeper"), the precision of the supramolecular drugs can bind to Aβ, cross the BBB and initiate the process of removing toxic species from the brain, according to the team, restoring the vasculature's waste-clearing function.
This offers hope for developing effective clinical interventions to address vascular contributions to Alzheimer's disease and ultimately improve patient outcomes, according to the team.
More research is needed to see how the findings translate in humans. "The BBB serves a similar role for all of us. If we can safely trigger the same recovery of barrier function in people, we expect improved brain housekeeping: steadier nutrient delivery, reduced inflammation and more effective clearance of toxic proteins. That combination could slow disease progression and boost the impact of other treatments," said Battaglia.