Forbes contributors publish independent expert analyses and insights.
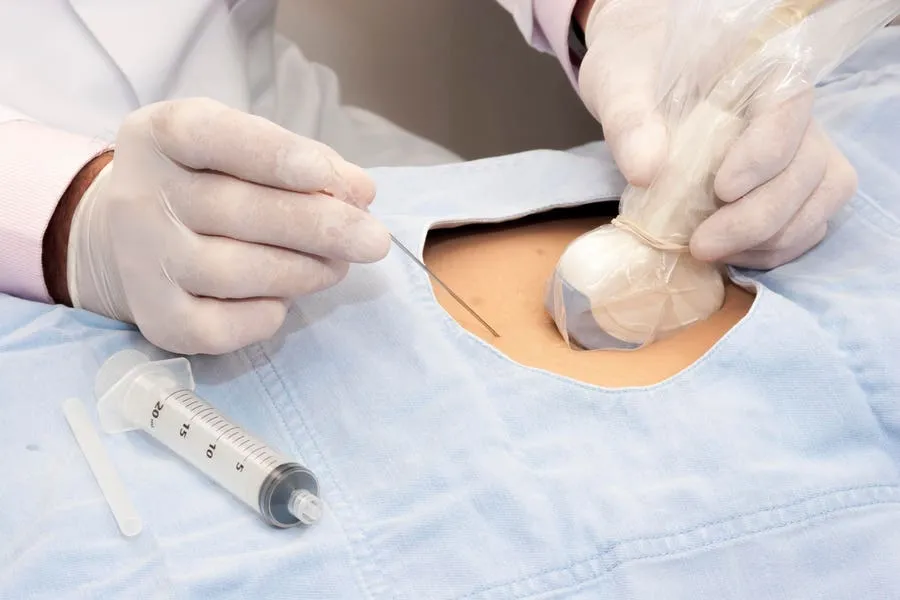
Healthcare robotics pioneer Mendaera announced today that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its handheld robotic system, Focalist, which aims to enhance ultrasound guided needle placement across a variety of medical and healthcare specialties. Specifically, the system integrates the use of a handheld module, real-time ultrasound imaging and proprietary software to simplify needle positioning, depth-tracking and placement to enable simplicity, consistency and reproducibility in needle based procedures.
Why is this important?
Image-guided needle placement is one of the most fundamental procedures across numerous specialties, including for accessing vital organs (e.g., for biopsies), establishing vascular access (e.g., central or arterial lines) and for managing pain and anesthesia (e.g., sedation for procedures). Furthermore, these procedures often require an incredible amount of patience, coordination, spatial perception and training to execute correctly, introducing a wide spectrum in the quality of care provided. The point of technologies such as Focalist is to introduce consistency and standardization in care experiences.
In fact, nearly 9 million biopsy procedures were conducted in 2022 alone. A study published in The Journal of the Missouri State Medical Association found that "Using modern tools to incorporate procedural skills training and ultrasound simulation" can vastly improve "clinical practice and decision-making by increasing confidence and competence with ultrasound needle-guided procedures." Furthermore, it describes the importance of mastering competency in procedural elements, such as needle placement, as a critical aspect of modern medicine; precision with these techniques is ultimately paramount to reducing the risk of misplacement and complications. Another study, published in Photoacoustics, describes how "Accurate needle guidance is crucial for safe and effective clinical diagnosis and treatment procedures" and also explains that needle insertion procedures are frequently victim to "challenges in consistency and precise visualization of the needle."
Indeed, optimizing for time efficiency, precision and consistent experiences across the millions of procedures done annually at health systems introduces a massive burden on a healthcare workforce that is already stretched thin. This is where technology such as Mendaera's can truly add value -- as a means to enable consistency and perhaps alleviate workflow burdens by making each procedure more streamlined.
The company is initially focusing on applications in urology, a speciality that is known for a high frequency of these procedures. Dr. Gerhard J. Fuchs, Professor of Clinical Urology at the University of Southern California, explains that "Simplifying ultrasound-guided percutaneous access is one of the most impactful ways to improve efficiency, safety, and independence in urology and beyond...Mendaera's platform represents a meaningful evolution - one with the potential to broaden access to minimally invasive procedures and elevate the standard of care."
Of course, there is a significant amount of work being done and competition in this field by a variety of different players. For example, in academic settings, scientists are trying to leverage deep learning and artificial intelligence to enhance needle visibility and improve image and anatomy detection. Other academics recognize the lack of data available to train AI models to assist in needle and target detection, and hence are working on understanding how synthetic data can instead be leveraged to train these systems. With regards to specifically the needle-based intervention space, XACT Robotics was granted FDA clearance in 2022, but eventually shutdown due to a variety of market challenges. Another prominent player is Interventional Systems, which received 510(k) FDA clearance in 2023 for its percutaneous CT navigation system, and has since continued to expand its presence in the market.
Peter Hebert, partner and co-founder of Lux Capital, which is a key investor in Mendaera, explains that "AI, real-time imaging, and innovative hardware can help bring the substantial benefits of medical robotics to mainstream procedures." He also addresses a central pain point: "As healthcare faces growing labor shortages and cost constraints, technology can be employed to enhance patient experiences while improving the efficiency of health systems. Mendaera's Focalist system is uniquely positioned to deliver the benefits of robotics - namely precision, consistent quality and efficiency - more broadly across healthcare."
Despite a challenging landscape, the competition has forced rapid innovation. Nevertheless, there need not be only one winner; instead, the space is certainly wide enough for multiple players to work towards improving both workforce burdens and consistency in the patient experience.